

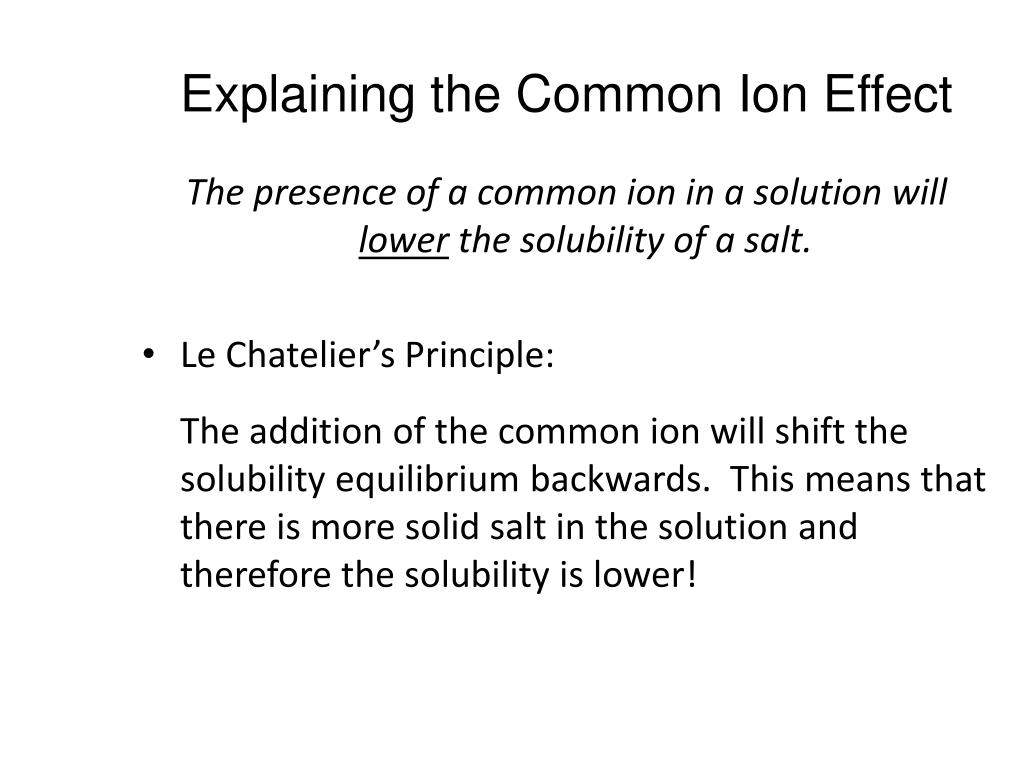
How Does a Solution Form? As a solution forms, the solvent pulls solute particles apart and surrounds, or solvates, them. Solutions The intermolecular forces between solute and solvent particles must be strong enough to compete with those between solute particles and those between solvent particles. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. (view Glencoe animation)Īn electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. In a solution, the solute is dispersed uniformly throughout the solvent.Solutions are homogeneous mixtures of two or more pure substances.If you can’t, the substance didn’t dissolve, it reacted.
Dissolution is a physical change-you can get back the original solute by evaporating the solvent.Just because a substance disappears when it comes in contact with a solvent, it doesn’t mean the substance dissolved. 2/animations_center.html# (dissolution of an ionic and a covalent compound osmosis) These summaries are to be included in your portfolio. Independent work - students to view animations & interactive activities (from Norton and from the Glencoe site for Chang’s book) and write summary notes on each. 13 – content required for regents (in part), SAT II and AP exams Lab activities: – Solubility of KNO3 – Colligative properties lab POGILS (6 – Some of these were done with Chapter 4 work) – – – – – –Ĭhapter 13 – sample and practice exercises, GIST, VC problems, selection of end of chapter exercises. Chem guy video-lectures at ses/ap-chemistry-withchemguy/video-lectures/ SolutionsĪctivities and Problem set for chapter 13 (due date_).aspx Chapter 12 Animations from glencoe website for Chang’s book: 0/chapter12/animations_center.html# Norton chapter 11 : mistry/chemistr圓/ch/11/chemtours. Molarity Saturated and Unsaturated Solutions Solubility Common Ion Effect on Solubility Fractional Precipitation Solution StoichiometryĬhemtour videos from W.W. Textbook - chapter 13 & ppt file (AP, SAT II and regents exams).(This ppt is a modified file from our Textbook publisher with additional slides taken from ppt file found at ) aspects of this topic were seen in chapter 4) BurstenĬhapter 13 (part I of II)Properties of Solutions (N.B. Chemistry, The Central Science, 10th edition, AP version Theodore L.


 0 kommentar(er)
0 kommentar(er)
